
「キラリティー」は、様々な物質の性質を進展させる要素です。物質にキラリティーを組み込むことは、その機能の飛躍的な向上に繋がり、夢の物質を創り出す第一歩となります。私たちの生活に欠かすことのできない物質・材料にキラリティーを組み込むこと、それを可能にする一連の方法論を開発することは、次世代の純粋化学と応用化学の両面、そして材料科学において極めて大きな意味を持ちます。
私たちは、キラル機能性物質開発への応用展開を最終目標に、現在、その基盤づくりに取り組んでいます。独自のキラル分子をデザインし、独自の反応・手法の開発を通じて、独自のキラル分子の合成を進めています。様々な解析手法を駆使して分子の振る舞いを理解し、従来とは異なる分子の性質を見出すことで、新たな機能を有するキラル物質を創り出していきたいと考えています。

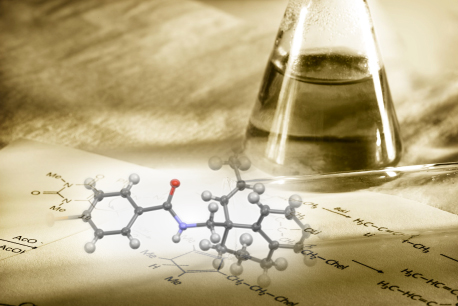
世の中には、多彩な分子変換反応が存在しています。種々の分子変換により、多様な分子が生み出されています。先人たちの反応への「こだわり」が、様々な分子変換を実現してきたのです。私たちも独自の反応の開発に「こだわり」ます。私たちの「こだわりの反応」が、いつしか新たな分子を合成するための一手となること、それが、私たちの願いです。

私たちの生活は、銘々の「こだわり」が詰まった建物において営まれています。匠が「こだわりの技法」により「こだわりの建物」を築いていくように、私たちも独自に開発した「こだわり手法」により、オリジナルの分子を創ることに「こだわり」ます。私たちの「こだわりの分子」が、いつしか新たな機能の発現の一助となること、それが、私たちの願いです。


Communication and Full paper
- 39.
- “TBA”
Ohtsuka, N.; Kotani, S.; Fujinami, T.; Sugiura, S.; Suzuki, T.; Momiyama, N.
under preparation for submission
- 38.
- “TBA”
Fujinami, T.; Suzuki, T.; Momiyama, N.
submitted
- 37.
- “TBA”
Kato, M.; Momiyama, N. et al.
under preparation for submission
- 36.
- “TBA”
Oishi, S.; Ohtsuka, N.; Maruyama, R.; Mendoza Zamarripa, E. M.; Irie, M.; Mase, N.; Suzuki, T.; Momiyama, N.
ChemRxiv
10.26434/chemrxiv-2025-1r6gz/v2
- 35.
-
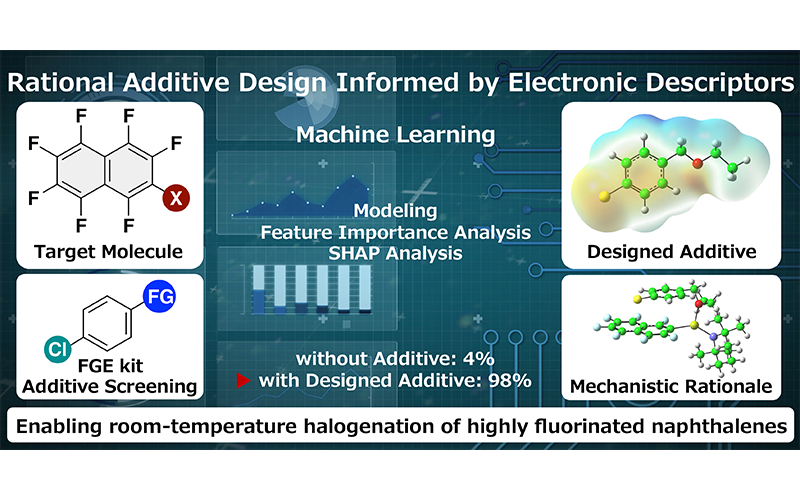
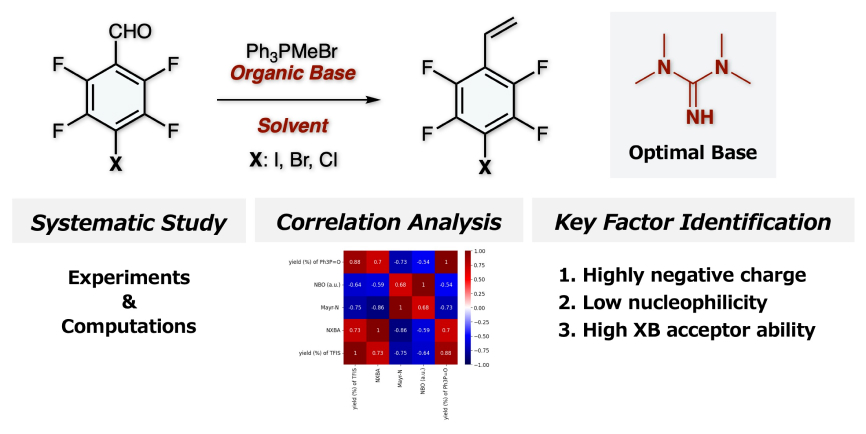
“Data-guided rational design of additives for halogenation of highly fluorinated naphthalenes: integrating fluorine chemistry and machine learning”
Ohtsuka, N.; Mohd Aris, Z. M.; Suzuki, T.; Momiyama, N.
Phys. Chem. Chem. Phys. in press
DOI: 10.1039/d5cp03554f
- 34.
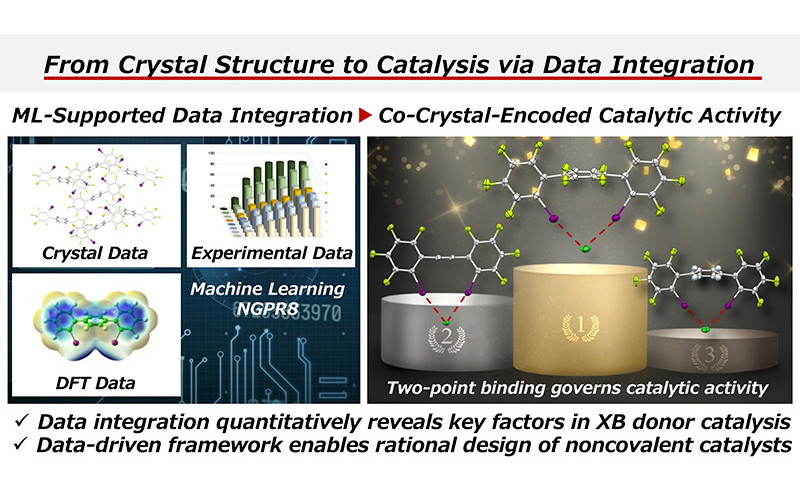
- “Data-Integrated Elusidation of Structure–Activity Relationships toward the Rational Design of Perfluoiodoarene-Based Halogen-Bond Donor Catalysts”
Kato, M.; Nakashima, F.; Ohtsuka, N.; Nishioka, Y.; Izumiseki, A.; Fujinami, T.; Oishi, S.; Suzuki, T.; Momiyama, N.
J. Org. Chem. 2026, 91, 2088–2104.
DOI: 10.1021/acs.joc.5c02704
- 33.
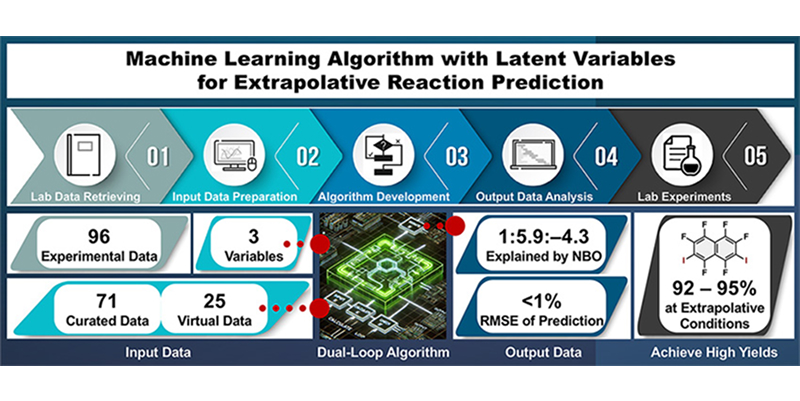
- “Machine learning-guided synthesis of prospective organic molecular materials: An algorithm with latent variables for understanding and predicting experimentally unobservable reactions”
Takeda, K.; Ohtsuka, N.; Suzuki, T.; Momiyama, N.
Artif. Intell. Chem.
2025,
3, 100096.
DOI: 10.1016/j.aichem.2025.100096
分子科学研究所プレスリリース
https://www.ims.ac.jp/news/2025/11/1121.html
Web報道
https://tiisys.com/blog/2026/01/21/post-183805/
https://tiisys.com/blog/2025/11/21/post-180348/
http://www.360doc.com/content/25/1123/17/8176916_1165405372.shtml
https://www.techeyesonline.com/news/detail/eetimesjapan-202511261530-1/
https://eetimes.itmedia.co.jp/ee/articles/2511/26/news033.html
- 32.
- “Prediction Method for Reactiopn Yield of Deuteration of Polyfluoroperylene using Generative AI Techniques”
Takeda, K.; Ohtsuka, N.; Suzuki, T.; Momiyama, N.
Comput. Aided Chem. Eng.
2024,
53, 2689–2694.
DOI: 10.1016/B978-0-443-28824-1.50449-X
- 31.
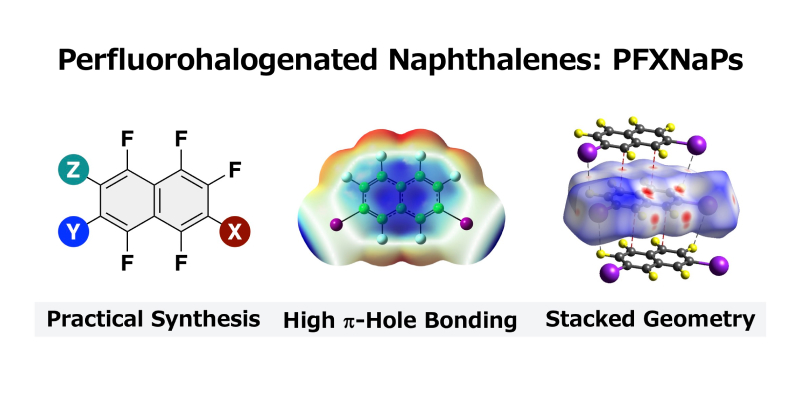
- “Perfluorohalogenated naphthalenes: synthesis, crystal structure, and intermolecular interaction”
Ohtsuka, N.; Ota, H.; Sugiura, S.; Kakinuma, S.; Sugiyama, H.; Suzuki, T.; Momiyama, N.
CrystEngComm,
2024,
26, 764–772.
DOI: 10.1039/d3ce01124k
- 30.
- “Synthesis of Halogen Bond Donor Site-Introduced Functional Monomers via Wittig Reaction of Perfluorohalogenated Benzaldehydes: Toward Digitalization as Reliable Strategy in Small Molecule Synthesis”
Hori, T.; Kakinuma, S.; Ohtsuka, N.; Fujinami, T.; Suzuki, T.; Momiyama, N.
Synlett 2023, 34, 2455–2460.
(Special Issue Dedicated to Prof. Hisashi Yamamoto, invitation only).
DOI: 10.1055/a-2118-6813
- 29.
- “Mutational and Environmental Effects on the Dynamic Conformational Distributions of Lys48-Linked Ubiquitin Chains”
Hiranyakorn, M.; Yagi-Utsumi, M.; Yanaka, S.; Ohtsuka, N.; Momiyama, N.; Satoh, T.; Kato, K.
Int. J. Mol. Sci.
2023, 14, 2521-2523.
DOI: 10.3390/ijms24076075
- 28.
- “Protocol for efficient dearomatization of N-heteroaromatics with halogen(I) complex catalyst”
Oishi, S.; Fujinami, T.; Masui, Y.; Suzuki, T.; Kato, M.; Ohtsuka, N.; Momiyama, N.
STAR Protocols, 2023, 4, 102140.
DOI: 10.1016/j.xpro.2023.102140
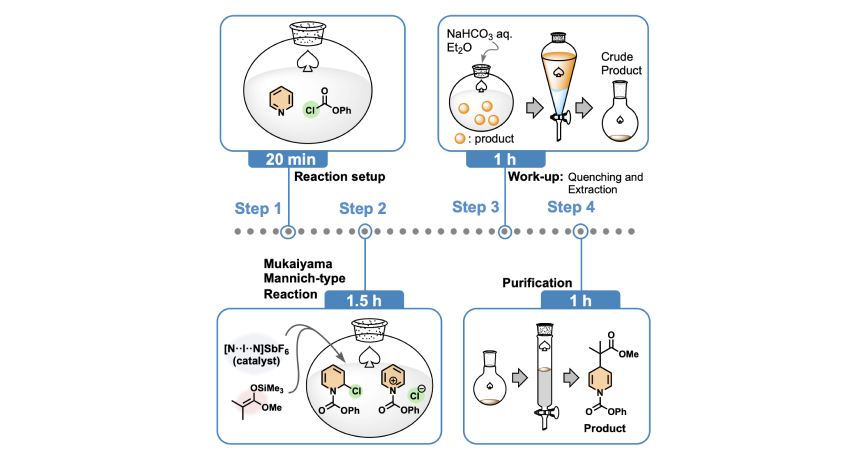
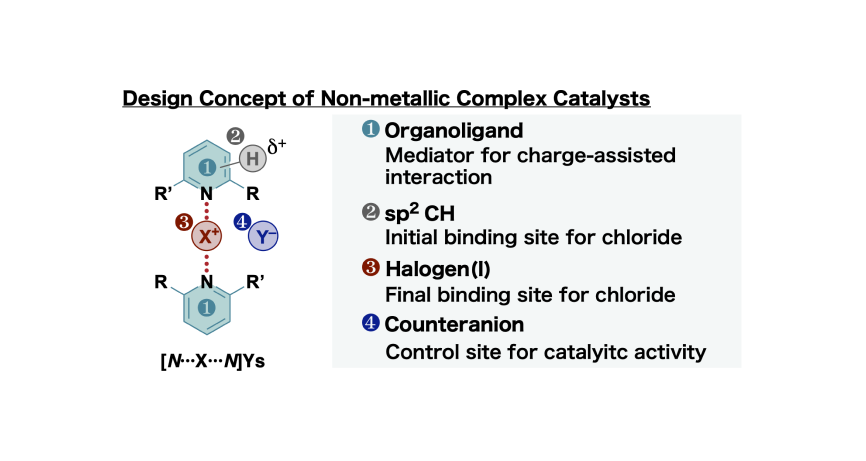
- 27.
- “Three-center-four-electron halogen bond enables non-metallic complex catalysis for
Mukaiyama–Mannich-type reaction”
Oishi, S.; Fujinami, T.; Masui, Y.; Suzuki, T.; Kato, M.; Ohtsuka, N.; Momiyama, N.
iScience,
2022, 25, 105220.
DOI:
10.1016/j.isci.2022.105220
分子科学研究所プレスリリース
https://www.ims.ac.jp/news/2022/10/1020.html
Web報道
https://news.biglobe.ne.jp/it/1021/mnn_221021_9362368362.html
https://news.mynavi.jp/techplus/article/20221021-2486850/
https://www.nikkei.com/prime/tech-foresight/article/DGXZQOUC106PF0Q2A111C2000000
https://xtech.nikkei.com/atcl/nxt/column/18/02122/00106/
https://www.chem-station.com/blog/2022/11/mmtr.html
学術誌での紹介
https://pubs.acs.org/doi/10.1021/jacs.3c11449
https://onlinelibrary.wiley.com/doi/10.1002/anie.202404823
https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/ejoc.202400213
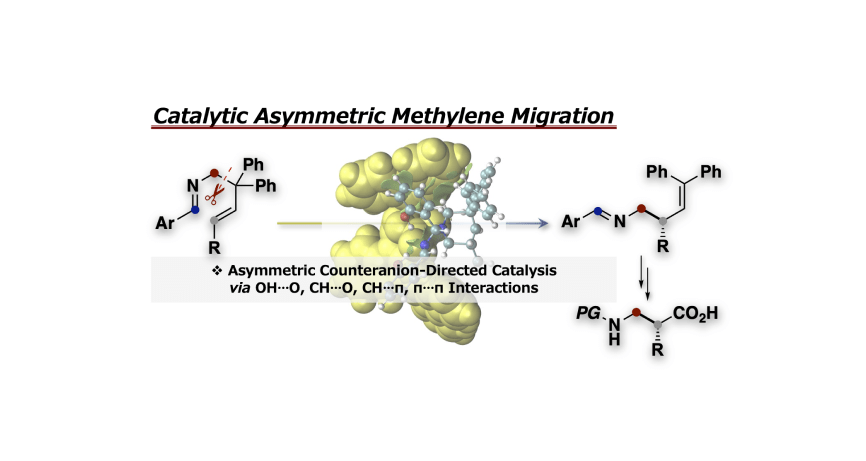
- 26.
- “Chiral Counteranion-Directed Catalytic Asymmetric Methylene Migration Reaction of Ene-Aldimines”
Momiyama, N.; Jongwohan, C.; Ohtsuka, N.; Chaibuth, P.; Fujinami, T.; Adachi, K.; Suzuki, T.
J. Org. Chem.
2022, 87, 9399-9407.
DOI: 10.1021/acs.joc.2c00742
学術誌での紹介
https://www.beilstein-journals.org/bjoc/articles/20/201
- 25.
- “Moderately Oxidizing Thioxanthylium Organophotoredox Catalysts for Radical-Cation Diels–Alder
Reactions”
Tanaka, K.; Kishimoto, M.; Tanaka, Y.; Kamiyama, Y.; Asada, Y.; Sukegawa, M.; Ohtsuka, N.; Suzuki, T.;
Momiyama, N.; Honda, K.; Hoshino, Y.
J. Org. Chem.
2022, 87, 3319-3328.
DOI:
10.1021/acs.orglett.1c02972
- 24.
- “Quasi-homoepitaxial Junction of Organic Semiconductors: A Structurally Seamless but Electronically
Abrupt Interface between Rubrene and Bis(trifluoromethyl)-dimethyl-rubrene”
Takahashi, K.; Izawa, S.; Ohtsuka, N.; Izumiseki, A.; Tsuruta, R.; Takeuchi, R.; Gunjo, Y.; Nakanishi,
Yu.; Mase, K.; Koganezawa, T.; Momiyama, N.; Hiramoto, M.; Nakayama, Y.
J. Phys. Chem. Lett.
2021,
12, 11430-11437.
DOI:
10.1021/acs.jpclett.1c03094
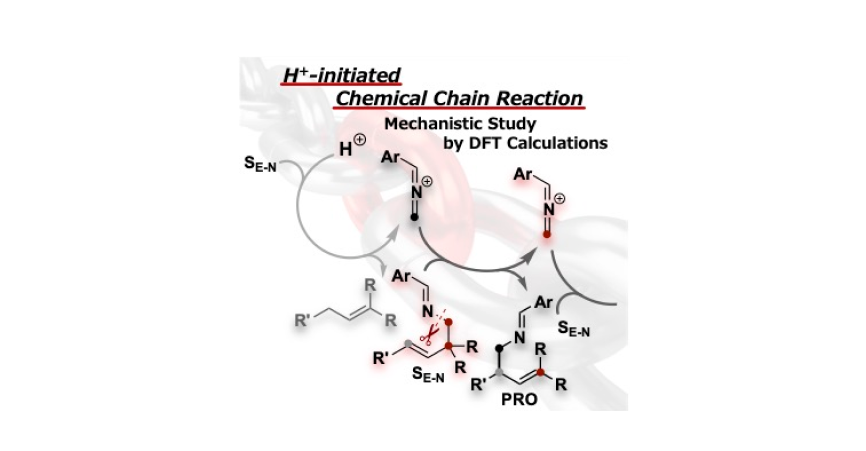
- 23.
- “Computational Studies on Reaction Mechanisms and Origin of Stereoselectivity in the [1,3]-Rearrangement
of Ene-Aldimines”
Momiyama, N.; Honda, Y.; Suzuki, T.; Jongwohan, C.
Asian JOC
2021,
10, 2205-2112 (Special Issue for Organocatalysis, invitation only).
DOI: 10.1002/ajoc.202100302
- 22.
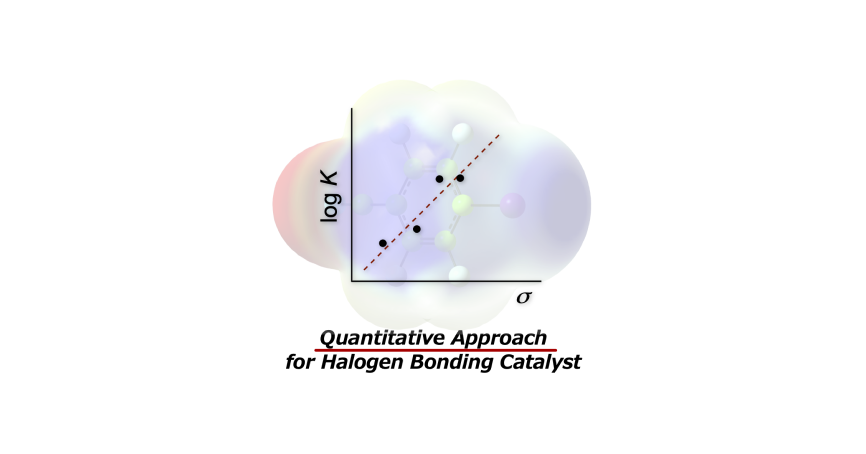
- “Correlations between Substituent Effects and Catalytic Activities: A Quantitative Approach for the
Development of Halogen-Bonding-Driven Anion-Binding Catalysts”
Momiyama, N.; Izumiseki, A.; Ohtsuka, N.; Suzuki, T.
ChemPlusChem
2021, 86, 913-919 (Special Issue for ISXB-4,
invitation only).
DOI: 10.1002/cplu.202100147
- 21.
- “Brønsted Acid-Initiated Formal [1,3]-Rearrangement Dictated by β-Substituted Ene-Aldimines”
Jongwohan, C.; Honda, Y.; Suzuki, T.; Fujinami, T.; Adachi, K.; Momiyama, N.
Org. Lett. 2019, 21, 4991-4995.
DOI:
10.1021/acs.orglett.9b01533
- 20.
- “Molecular Design of a Chiral Brønsted Acid with Two Different Acidic Sites: Regio-, Diastereo-, and
Enantioselective Hetero-Diels–Alder Reaction of Azopyridinecarboxylate with Amidodienes Catalyzed by
Chiral Carboxylic Acid–Monophosphoric Acid”
Momiyama, N.; Tabuse, H.; Noda, H.; Yamanaka, M.; Fujinami, T.; Yamanishi, K.; Izumiseki, A.; Funayama,
K.; Egawa, F.; Okada, S.; Adachi, H.; Terada, M.
J. Am. Chem. Soc. 2016, 138, 11353-11359.
DOI: 10.1021/jacs.6b07150
Highlighted in Synfacts 2016, 12, 1198.
DOI: 10.1055/s-0036-1589421
- 19.
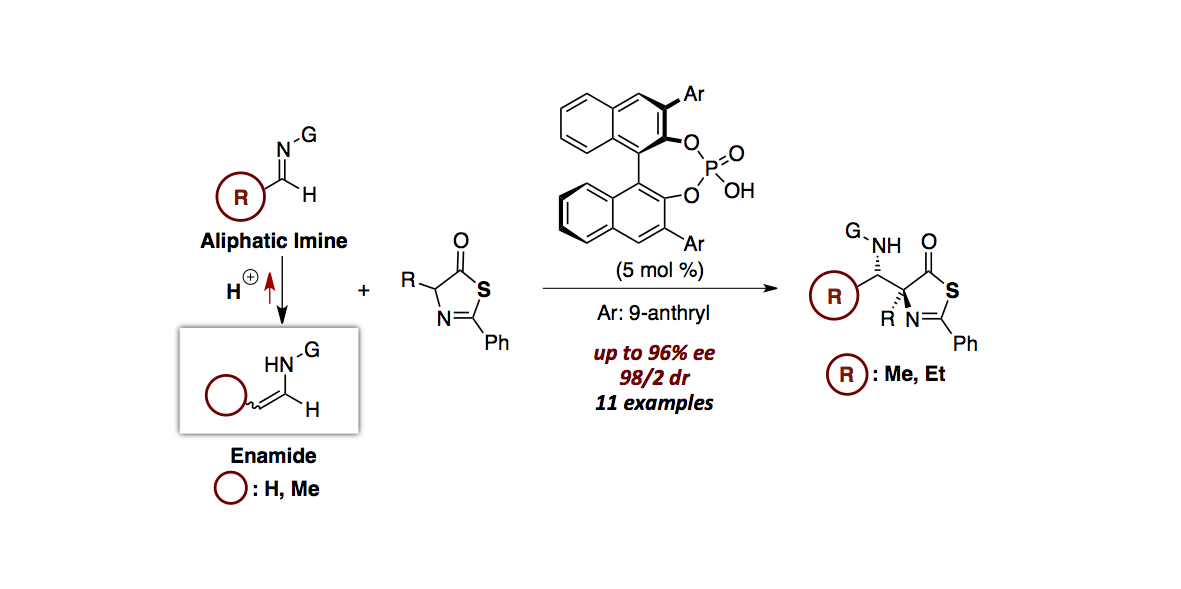
- “Chiral Phosphoric Acid-Catalyzed Diastereo- and Enantioselective Mannich-Type Reaction between Enamides
and Thiazolones”
Kikuchi, J.; Momiyama, N.; Terada, M.
Org. Lett. 2016, 18, 2521-2523.
DOI:
10.1021/acs.orglett.6b00857
- 18.
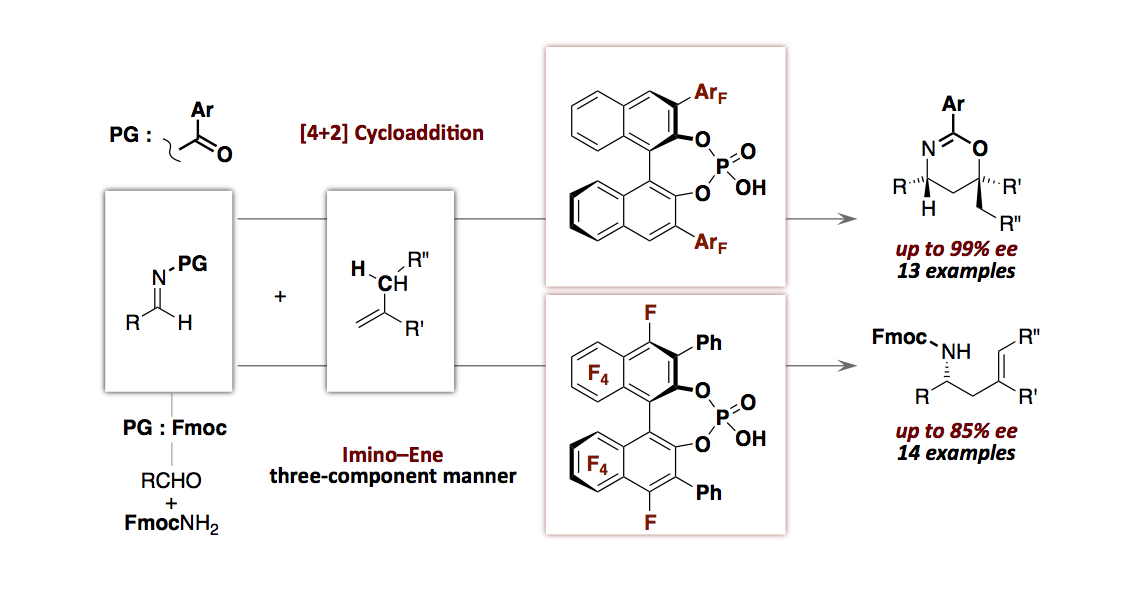
- “Perfluorinated Aryls in the Design of Chiral Brønsted Acid Catalysts: Catalysis of Enantioselective
[4+2] Cycloadditions and Ene–Reactions of Imines with Alkenes by Chiral Mono-Phosphoric Acids with
Perfluoroaryls”
Momiyama, N.; Okamoto, H.; Kikuchi, J.; Korenaga, T.; Terada, M.
ACS Catal. 2016, 6, 1198-1204.
DOI:10.1021/acscatal.5b02136
Highlighted in Synfacts 2016, 12, 413.
DOI:10.1055/s-0035-1561838
- 17.
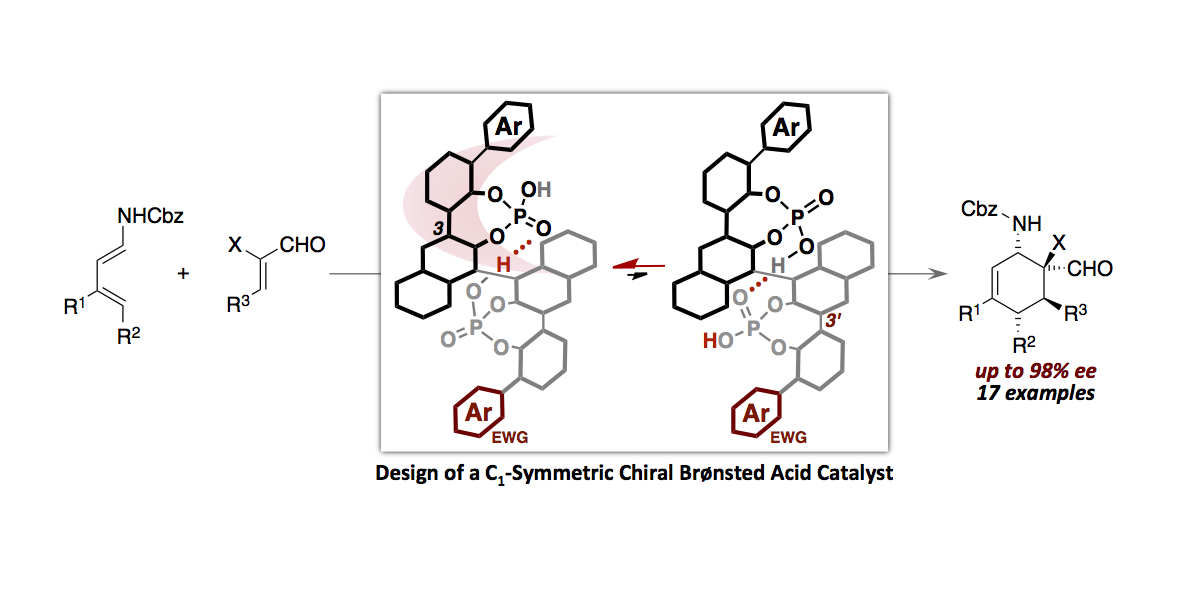
- “Hydrogen Bonds-Enabled Design of a C1-Symmetric Chiral Brønsted Acid Catalyst”
Momiyama, N. Funayama, K.; Noda, H.; Yamanaka, M.; Akasaka, N.; Ishida, S.; Iwamoto, T.; Terada, M.
ACS Catal. 2016, 6, 949-956.
DOI: 10.1021/acscatal.5b02079
Highlighted in Synfacts. 2016, 12, 308.
DOI: 10.1055/s-0035-1561676
- 16.
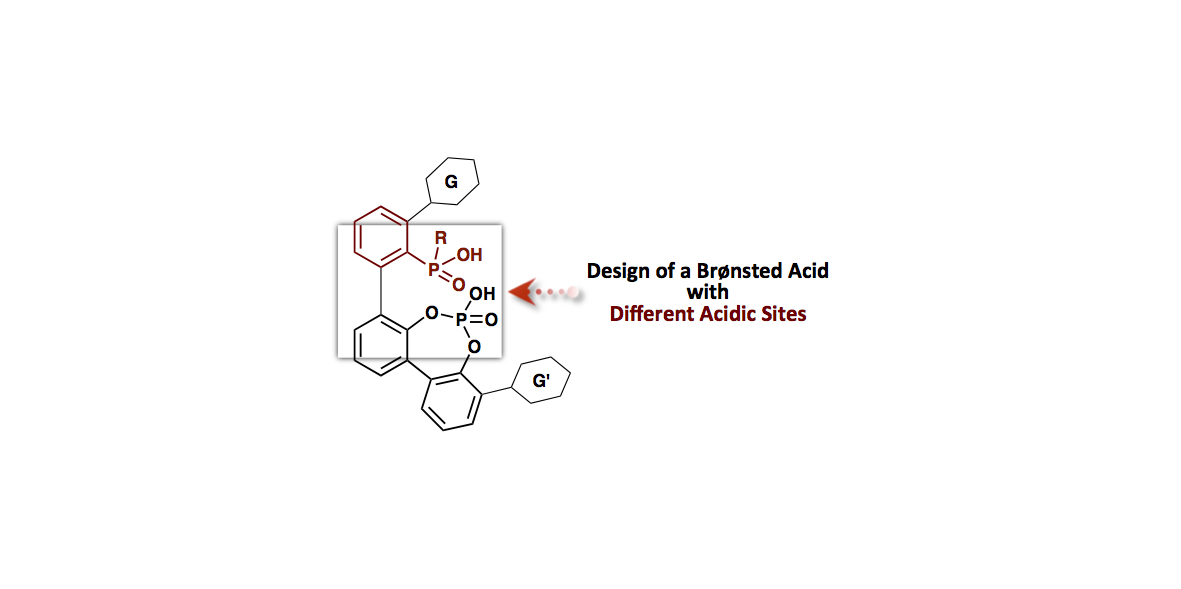
- “Design of a Brønsted Acid with Two Different Acidic Sites: Synthesis and Application of Aryl Phosphinic
Acid-Phosphoric Acid as a Brønsted Acid Catalyst”
Momiyama, N.; Narumi, T.; Terada, M.
Chem. Commun. 2015, 51, 16976-16979.
DOI: 10.1039/C5CC06787A
- 15.
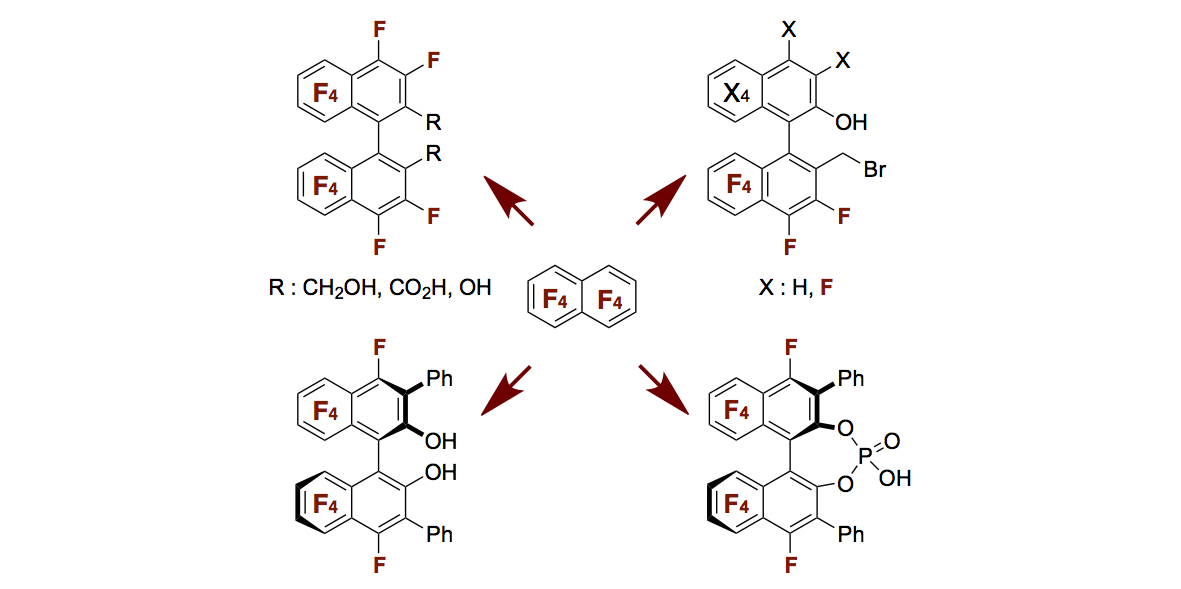
- “Synthetic Method for 2,2’-Disubstituted Fluorinated Binaphthyl Derivatives and Application as Chiral
Source in Design of Chiral Mono-Phosphoric Acid Catalyst”
Momiyama, N.; Okamoto, H.; Shimizu, M.; Terada, M.
Chirality 2015,
27, 464-475 (Invited).
DOI: 10.1002/chir.22429
- 14.
- “Design of Chiral Bis-phosphoric Acid Catalyst Derived from (R)-3,3’-Di(2-hydroxy-3-arylphenyl)binaphthol: Catalytic Enantioselective
Diels-Alder Reaction of α,β-Unsaturated Aldehydes with Amidodienes”
Momiyama, N.; Konno, T.; Furiya, Y.; Iwamoto, T.; Terada, M.
J. Am. Chem. Soc. 2011, 133, 19294-19297.
DOI: 10.1021/ja2081444
- 13.
- “Chiral Brønsted Acid Catalysis for Enantioselective Hosomi-Sakurai Reaction of Imines with
Allyltrimethylsilanes”
Momiyama, N.; Nishimoto, H.; Terada, M.
Org. Lett. 2011, 13, 2126-2129.
DOI: 10.1021/ol200595b
- 12.
- “Chiral Phosphoric Acid-Governed Anti-Diastereoselective and Enantioselective Hetero-Diels-Alder
Reaction of Glyoxylate”
Momiyama, N.; Tabuse, H.; Terada, M.
J. Am. Chem. Soc. 2009, 131, 12882-12883.
DOI: 10.1021/ja904749x
- 11.
- “Enantioselective Activation of Aldehydes by Chiral Phosphoric Acid Catalysts in an Aza-Ene-type
Reaction between Glyoxylate and Enecarbamate”
Terada, M.; Soga, K.; Momiyama, N.
Angew. Chem., Int. Ed. 2008, 47, 4122-4125.
DOI: 10.1002/anie.200800232
- 10.
- “Synthesis of Acyclic α,β-Unsaturated Ketones via Pd(II)-Catalyzed
Intermolecular Reaction of Alkynamides and Alkenes”
Momiyama, N.; Kanan, M. W.; Liu, D. R.
J. Am. Chem. Soc. 2007, 129, 2230-2231.
DOI: 10.1021/ja068886f
- 9.
- “Diastereo- and Enantioselective Synthesis of Nitroso Diels-Alder-type Bicycloketones Using Dienamine:
Mechanistic Insight into Sequential Nitroso Aldol/Michael Reaction and Application for Optically Pure
1-Amino-3,4-diol Synthesis”
Momiyama, N.; Yamamoto, Y.; Yamamoto, H.
J. Am. Chem. Soc. 2007, 129, 1190-1195.
DOI: 10.1021/ja066037m
- 8.
- “Metal-Induced Reaction of O-Nitroso Aldol Product”
Morales, M. R.; Momiyama, N.; Yamamoto, H.
Synlett 2006, 705-706.
DOI: 10.1055/s-2006-933123
- 7.
- “Brønsted Acid Catalysis of Achiral Enamine for Regio- and Enantioselective Nitroso Aldol Synthesis”
Momiyama, N.; Yamamoto, H.
J. Am. Chem. Soc. 2005, 127, 1080-1081.
DOI: 10.1021/ja0444637
- 6.
- “O-Nitroso Aldol Synthesis. Catalytic Enantioselective Route to α-Aminooxy Carbonyl Compounds via
Enamine Intermediate”
Momiyama, N.; Torii, H.; Saito, S.; Yamamoto, H.
Proc. Natl. Acad. Sci. U.S.A. 2004, 101, 5374-5378.
DOI: 10.1073/pnas.0307785101
- 5.
- “Enantioselective Tandem O-Nitroso Aldol/Michael Reaction”
Yamamoto, Y; Momiyama, N.; Yamamoto, H.
J. Am. Chem. Soc. 2004, 126, 5962-5963.
DOI: 10.1021/ja049741g
- 4.
- “Enantioselective O- and N-Nitroso Aldol Synthesis of Tin Enolates. Isolation of Three BINAP-Silver
Complexes and Their Role of in Regio- and Enantioselectivity”
Momiyama, N.; Yamamoto, H.
J. Am. Chem. Soc. 2004, 126, 5360-5361.
DOI: 10.1021/ja039103i
- 3.
- “Catalytic Enantioselective Synthesis of α-Aminooxy and α-Hydroxy Ketone Using Nitrosobenzene”
Momiyama, N.; Yamamoto, H.
J. Am. Chem. Soc. 2003, 125, 6038-6039.; 2004, 126, 6498.
DOI: 10.1021/ja0298702,
10.1021/ja040805x
- 2.
- “Simple Synthesis of α-Hydroxyamino Carbonyl Compounds: New Scope of the Nitroso Aldol Reaction”
Momiyama, N.; Yamamoto, H.
Org. Lett. 2002, 4, 3579-3582.
DOI: 10.1021/ol026443k
- 1.
- “Lewis Acid Promoted, O-Selective, Nucleophilic Addition of Silyl Enol Ethers to N=O bonds”
Momiyama, N.; Yamamoto, H.
Angew. Chem., Int. Ed. 2002, 41, 2986-2988.; 3313.
DOI:
10.1002/1521-3773(20020816)41:16<2986::AID-ANIE2986>3.0.CO;2-F,
10.1002/1521-3773(20020916)41:18<3313::AID-ANIE11113313>3.0.CO;2-X
Review and Book
- 7.
- “三中心ハロゲン結合を基盤とする分子性触媒の創成−有機配位子と非金属活性中心の組み合わせ−”
椴山 儀恵
2022年58巻10号p.943-947.
https://doi.org/10.14894/faruawpsj.58.10_948
- 6.
- “Noncovalent Interactions in the Design of Chiral Brønsted Acid Catalysts”
Momiyama, N.
Chapter 10, pp. 209-231. In Noncovalent Interactions in Catalysis, Mahmudov,
K. T.; Kopylovich, M. N.; Guedes da Silva, M-F. C.; Pombeiro, A-J. L. Eds. The Royal Society of Chemistry
2019.
https://pubs.rsc.org/en/Content/eBook/978-1-78801-468-7
- 5.
- “Enantioselective Synthesis of Amines by Chiral Brønsted Acid Catalysts”
Terada, M.; Momiyama, N.
pp. 75-129, Chiral Amine Synthesis, Nugent, T. C. Ed. 2010.
- 4.
- “Development of Organocatalysis based on the Molecular Design of Pyrrolidine-Brønsted Acid”
Saito, S.; Momiyama, N.; Yamamoto, H.
Yuki Gosei Kagaku Kyokaishi, 2008, 66, 774-784.
- 3.
- “Brønsted Acid Catalyzed Nitroso Aldol Reaction”
Momiyama, N.; Yamamoto, H.
Chapter 8, pp. 85-105. CMC books, Shibasaki, M. Ed. 2006.
- 2.
- “Nitrosobenzene”
Momiyama, N.
Electronic Encyclopedia of Reagents for Organic Synthesis.
- 1.
- “Rich Chemistry of Nitroso Compounds”
Yamamoto, H.; Momiyama, N.
Chem. Commun. 2005, 3514-3525.
Patents
- 7.
- “ピリジン誘導体担持高分子及びこれを用いた転移反応”
 儀恵、山西克典、渡辺拓 特開2019−189808(P2019−189808A)
儀恵、山西克典、渡辺拓 特開2019−189808(P2019−189808A)
- 6.
- “環上に置換基を有する含窒素環状化合物の製造方法”
 儀恵、泉関督人 特開2018−104361(P2018-104361A)
儀恵、泉関督人 特開2018−104361(P2018-104361A)
- 5.
- “Process for preparation of bisphosphosphates as catalysts for asymmetric reactions”
Terada, M.; Momiyama, N.; Konno, T.
PCT Int. Appl. 2011, WO 2011111677 A1 20110915.
- 4.
- “Process of making alpha-aminooxyketone/alpha-aminooxyaldehyde and alpha-hydroxyketone
/alpha-hydroxyaldehyde compounds and a process making reaction products from cyclic alpha,beta-unsaturated
ketone substrates and nitroso substrates.”
Momiyama, N.; Torii, H.; Saito, S.; Yamamoto, H.; Yamamoto, Y.
U.S. Pat. Appl. Publ. 2007, US20070037973 A1
20070215.
- 3.
- “Process of making α-aminooxyketone/α-aminooxyaldehyde compounds and α-hydroxyketone/α-hydroxyaldehyde
compounds and a process of making reaction products from cyclic α,s,s-unsaturated ketone substrates and
nitroso substrates.”
Momiyama, N.; Torii, H.; Saito, S.; Yamamoto, H.; Yamamoto, Y.
PCT Int. Appl. 2005, WO 2005090294 A2 20050929.
- 2.
- “Preparation of aminooxy compounds, hydroxy amines, and hydroxy ketones, and catalysis for it.”
Yamamoto, H.; Momiyama, N.
Jpn. Kokai Tokkyo Koho. 2004, JP 2004115446 A
20040415.
- 1.
- “Preparation of hydroxyamines and/or aminooxy compounds, and Lewis acid-containing catalysts for the
regioselecive nucleophilic addition reaction.”
Yamamoto, H.; Momiyama, N.; Yanagisawa, A.
Jpn. Kokai Tokkyo Koho. 2003, JP 2003313158 A
20031106.

![]()


 儀恵、山西克典、渡辺拓 特開2019−189808(P2019−189808A)
儀恵、山西克典、渡辺拓 特開2019−189808(P2019−189808A) 儀恵、泉関督人 特開2018−104361(P2018-104361A)
儀恵、泉関督人 特開2018−104361(P2018-104361A)