Research (Catalysis)
Aerobic oxidation of methanol on Au nano-particle
The chemical transformation of hydrocarbons catalyzed by nanometre-sized gold clusters has been of significant interest in industrial and academic research because of its remarkable potential for green chemistry and economic significance. Useful and practical reactions with gold catalysts have been extensively developed since the pioneering work of Haruta and co-workers. [Haruta, 1987]
We recently investigated (1) the aerobic oxidation of methanol to formic acid catalyzed by Au nanocluster supported by PVP [PCCP 2012] and (2) the homocoupling reaction catalyzed by Au/Pd:PVP alloy nanocluster [JACS 2012].
Bimetallic Au/Pd alloy nanoclusters (NCs) show the unique catalytic activity,
namely, Ullmann coupling of chloroarenes in aqueous media at low temperature.
The corresponding reaction cannot be achieved by monometallic Au and Pd
NCs as well as their physical mixtures. On the basis of quantum chemical
calculation, it was found that the crucial step to govern the unusual catalytic
activity of Au/Pd is the dissociative chemisorption of ArCl, which is unlikely
in the monometallic Au and Pd NCs. [JACS 2012]
(Joint work with Prof. H. Sakurai's group in IMS)
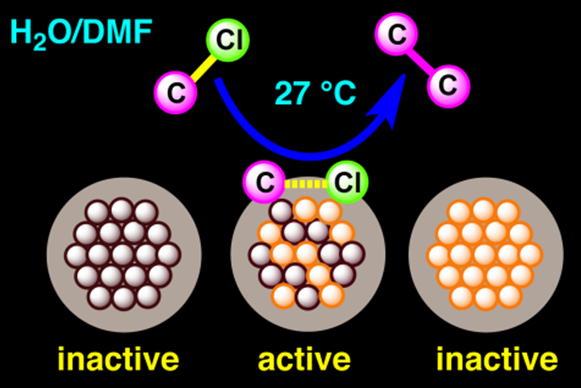
Ullmann coupling reaction catalyzed by Au/Pd:PVP
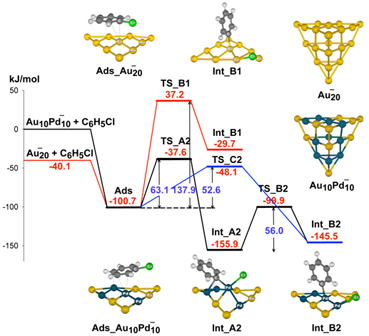
Energy diagram of the oxidative addition on Au and Au/Pd nanoclusters
The model systems Au20‒ and Au8‒ were adopted for the quantum chemical
calculations. The proposed reaction mechanism consists of three elementary
steps [PCCP 2012, IJQC 2012]: (1) formation of formaldehyde from methoxy
species activated by a superoxo-like anion on the gold cluster; (2) nucleophilic
addition by the hydroxyl group of a hydroperoxyl-like complex to formaldehyde
resulting in a hemiacetal intermediate; and (3) formation of formic acid
by hydrogen transfer from the hemiacetal intermediate to atomic oxygen
attached to the gold cluster. The catalytic cycles and energy diagram of
the catalytic reaction are shown in the figures.
(Joint work with Prof. H. Sakurai's group in IMS)
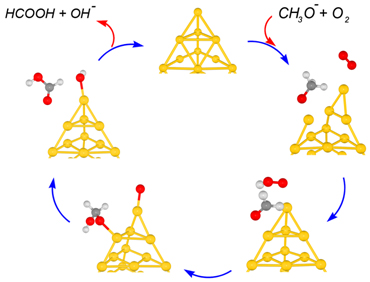
Catalytic cycles of aerobic oxidation of methanol on Au cluster
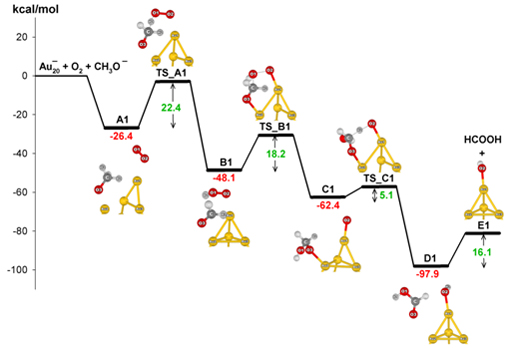
Energy diagram of aerobic oxidation of methanol on Au cluster

