5-1 Higashiyama, Myodaiji, Okazaki, 444-8787, Japan TEL: 0564-59-5531 


- 39.
- “TBA”
Ohtsuka, N.; Kotani, S.; Fujinami, T.; Sugiura, S.; Suzuki, T.; Momiyama, N.
under preparation for submission
- 38.
- “TBA”
Fujinami, T.; Suzuki, T.; Momiyama, N.
submitted
- 37.
- “TBA”
Kato, M.; Momiyama, N. et al.
under preparation for submission
- 36.
- “TBA”
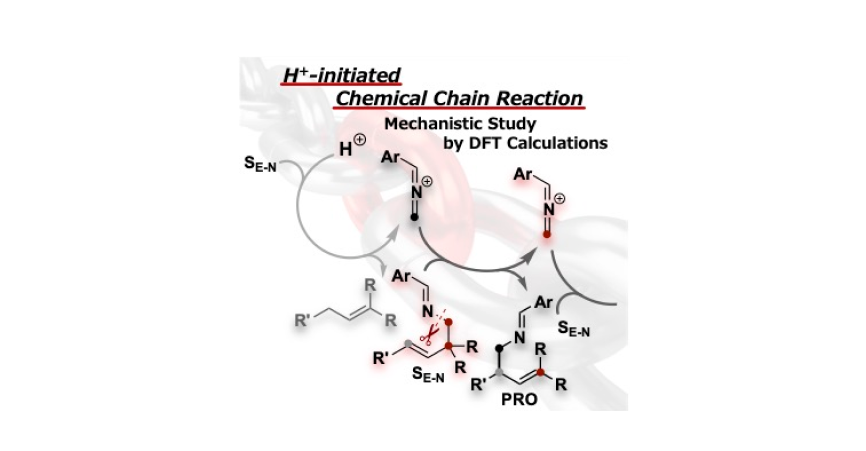
Oishi, S.; Ohtsuka, N.; Maruyama, R.; Mendoza Zamarripa, E. M.; Irie, M.; Mase, N.; Suzuki, T.; Momiyama, N.
ChemRxiv
DOI: 10.26434/chemrxiv-2025-1r6gz/v2
- 35.
-
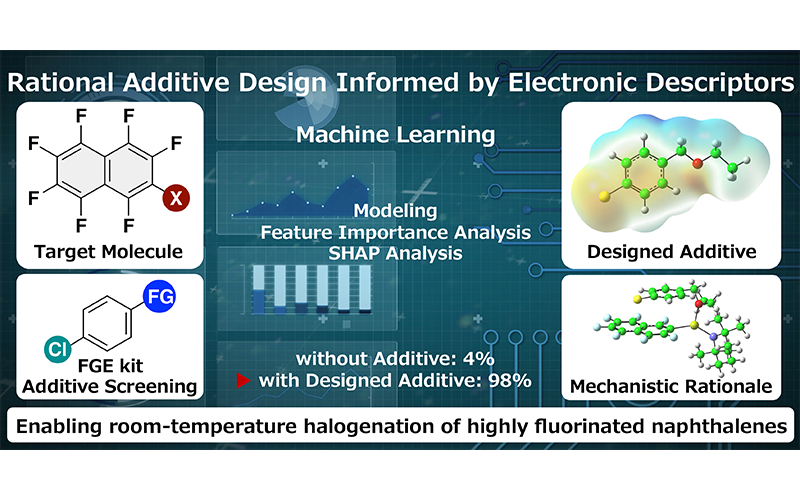
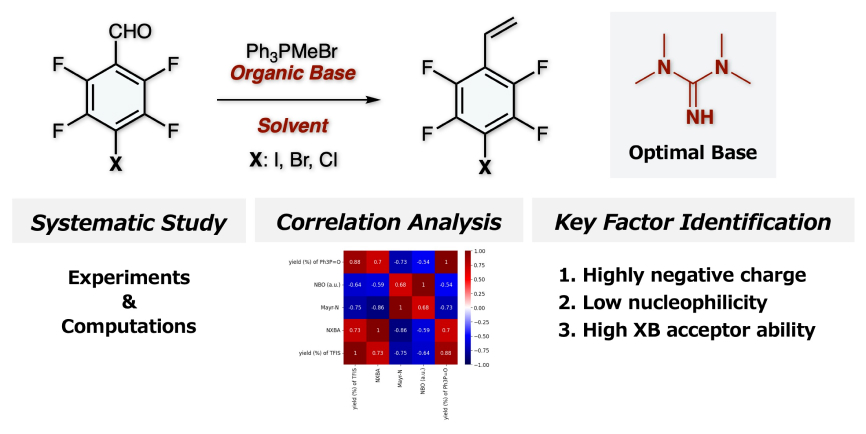
“Data-guided rational design of additives for halogenation of highly fluorinated naphthalenes: integrating fluorine chemistry and machine learning”
Ohtsuka, N.; Mohd Aris, Z. M.; Suzuki, T.; Momiyama, N.
Phys. Chem. Chem. Phys. in press
DOI: 10.1039/d5cp03554f
- 34.
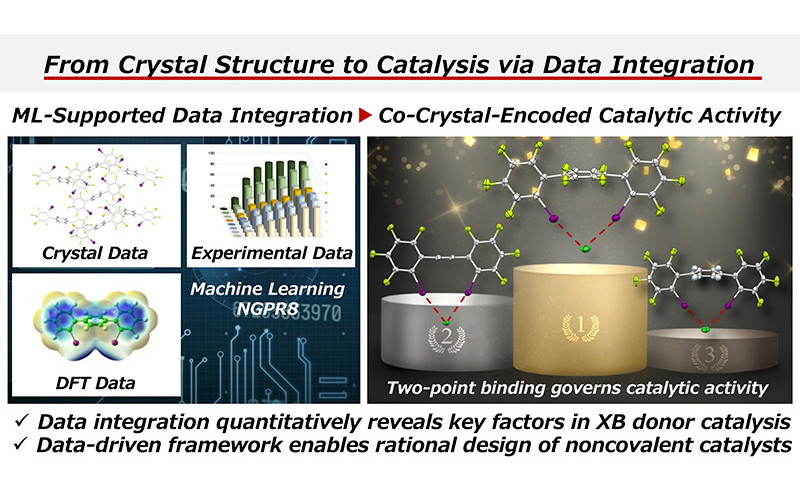
- “Data-Integrated Elusidation of Structure–Activity Relationships toward the Rational Design of Perfluoiodoarene-Based Halogen-Bond Donor Catalysts”
Kato, M.; Nakashima, F.; Ohtsuka, N.; Nishioka, Y.; Izumiseki, A.; Fujinami, T.; Oishi, S.; Suzuki, T.; Momiyama, N.
J. Org. Chem. 2026, 91, 2088–2104.
DOI: 10.1021/acs.joc.5c02704
- 33.
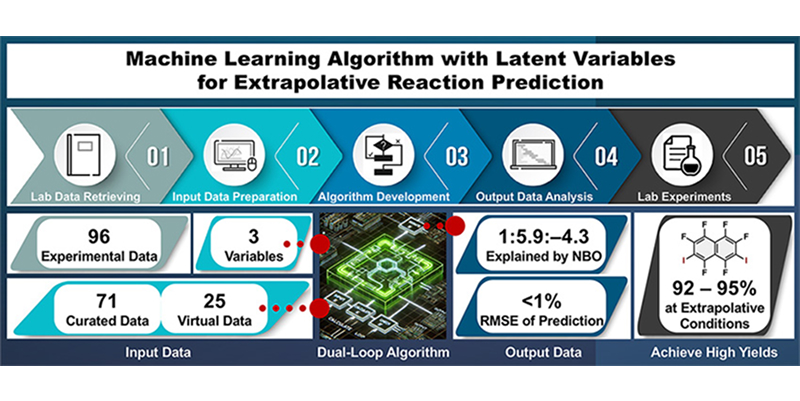
- “Machine learning-guided synthesis of prospective organic molecular materials: An algorithm with latent variables for understanding and predicting experimentally unobservable reactions”
Takeda, K.; Ohtsuka, N.; Suzuki, T.; Momiyama, N.
Artif. Intell. Chem.
2025,
3, 100096.
DOI: 10.1016/j.aichem.2025.100096
分子科学研究所プレスリリース
https://www.ims.ac.jp/news/2025/11/1121.html
Web報道
https://tiisys.com/blog/2026/01/21/post-183805/
https://tiisys.com/blog/2025/11/21/post-180348/
http://www.360doc.com/content/25/1123/17/8176916_1165405372.shtml
https://www.techeyesonline.com/news/detail/eetimesjapan-202511261530-1/
https://eetimes.itmedia.co.jp/ee/articles/2511/26/news033.html
- 32.
- “Prediction Method for Reactiopn Yield of Deuteration of Polyfluoroperylene using Generative AI Techniques”
Takeda, K.; Ohtsuka, N.; Suzuki, T.; Momiyama, N.
Comput. Aided Chem. Eng.
2024,
53, 2689–2694.
DOI: 10.1016/B978-0-443-28824-1.50449-X
- 31.
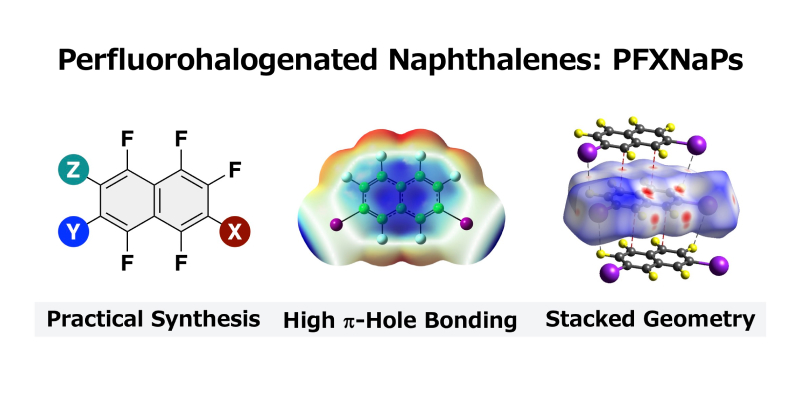
- “Perfluorohalogenated naphthalenes: synthesis, crystal structure, and intermolecular interaction”
Ohtsuka, N.; Ota, H.; Sugiura, S.; Sugiyama, H.; Suzuki, T.; Momiyama, N.
CrystEngComm,
2024,
26, 764–772.
DOI: 10.1039/d3ce01124k
- 30.
- “Synthesis of Halogen Bond Donor Site-Introduced Functional Monomers via Wittig Reaction of Perfluorohalogenated Benzaldehydes: Toward Digitalization as Reliable Strategy in Small Molecule Synthesis”
Hori, T.; Kakinuma, S.; Ohtsuka, N.; Fujinami, T.; Suzuki, T.; Momiyama, N.
Synlett 2023, 34, 2455–2460.
(Special Issue Dedicated to Prof. Hisashi Yamamoto, invitation only).
DOI: 10.1055/a-2118-6813
- 29.
- “Mutational and Environmental Effects on the Dynamic Conformational Distributions of Lys48-Linked Ubiquitin Chains”
Hiranyakorn, M.; Yagi-Utsumi, M.; Yanaka, S.; Ohtsuka, N.; Momiyama, N.; Satoh, T.; Kato, K.
Int. J. Mol. Sci.
2023, 14, 2521-2523.
DOI: 10.3390/ijms24076075
- 28.
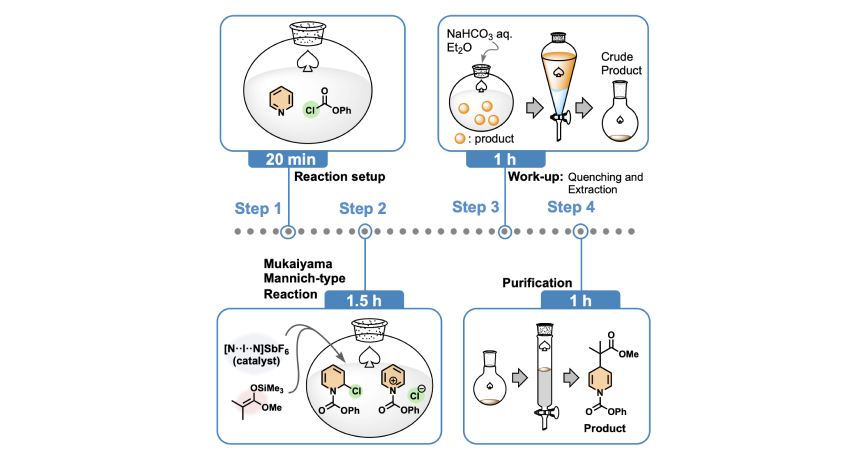
- “Protocol for efficient dearomatization of N-heteroaromatics with halogen(I) complex catalyst”
Oishi, S.; Fujinami, T.; Masui, Y.; Suzuki, T.; Kato, M.; Ohtsuka, N.; Momiyama, N.
STAR Protocols, 2023, 4, 102140.
DOI: 10.1016/j.xpro.2023.102140
- 27.
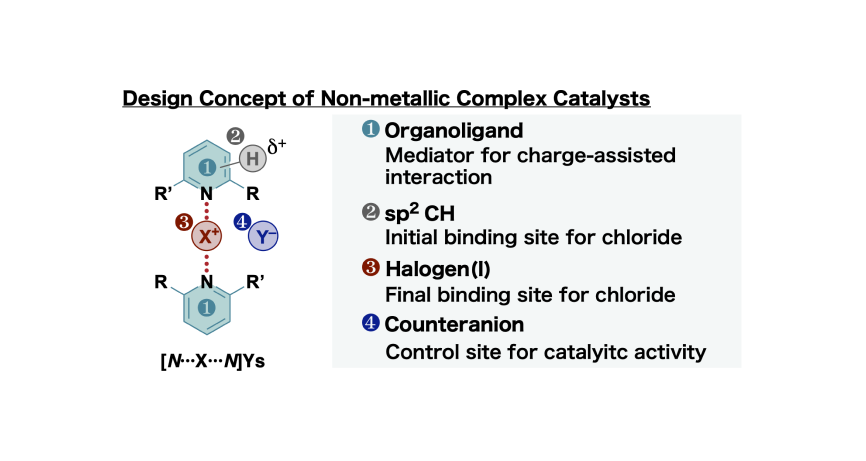
- “Three-center-four-electron halogen bond enables non-metallic complex catalysis for Mukaiyama–Mannich-type
reaction”
Oishi, S.; Fujinami, T.; Masui, Y.; Suzuki, T.; Kato, M.; Ohtsuka, N.; Momiyama, N.
iScience,
2022, 25, 105220.
DOI:
10.1016/j.isci.2022.105220
分子科学研究所プレスリリース
https://www.ims.ac.jp/news/2022/10/1020.html
Web報道
https://news.biglobe.ne.jp/it/1021/mnn_221021_9362368362.html
https://news.mynavi.jp/techplus/article/20221021-2486850/
https://www.nikkei.com/prime/tech-foresight/article/DGXZQOUC106PF0Q2A111C2000000
https://xtech.nikkei.com/atcl/nxt/column/18/02122/00106/
https://www.chem-station.com/blog/2022/11/mmtr.html
学術誌での紹介
https://pubs.acs.org/doi/10.1021/jacs.3c11449
https://onlinelibrary.wiley.com/doi/10.1002/anie.202404823
https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/ejoc.202400213
- 26.
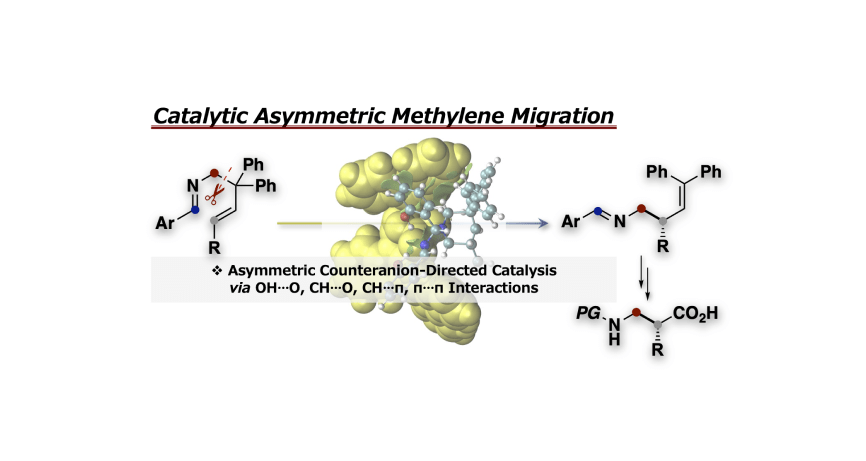
- “Chiral Counteranion-Directed Catalytic Asymmetric Methylene Migration Reaction of Ene-Aldimines”
Momiyama, N.; Jongwohan, C.; Ohtsuka, N.; Chaibuth, P.; Fujinami, T.; Adachi, K.; Suzuki, T.
J. Org. Chem.
2022, 87, 9399-9407.
DOI: 10.1021/acs.joc.2c00742
学術誌での紹介
https://www.beilstein-journals.org/bjoc/articles/20/201
- 25.
- “Moderately Oxidizing Thioxanthylium Organophotoredox Catalysts for Radical-Cation Diels–Alder
Reactions”
Tanaka, K.; Kishimoto, M.; Tanaka, Y.; Kamiyama, Y.; Asada, Y.; Sukegawa, M.; Ohtsuka, N.; Suzuki, T.;
Momiyama, N.; Honda, K.; Hoshino, Y.
J. Org. Chem.
2022, 87, 3319-3328.
DOI: 10.1021/acs.orglett.1c02972
- 24.
- “Quasi-homoepitaxial Junction of Organic Semiconductors: A Structurally Seamless but Electronically Abrupt
Interface between Rubrene and Bis(trifluoromethyl)-dimethyl-rubrene”
Takahashi, K.; Izawa, S.; Ohtsuka, N.; Izumiseki, A.; Tsuruta, R.; Takeuchi, R.; Gunjo, Y.; Nakanishi, Yu.;
Mase, K.; Koganezawa, T.; Momiyama, N.; Hiramoto, M.; Nakayama, Y.
J. Phys. Chem. Lett.
2021,
12, 11430-11437.
DOI:
10.1021/acs.jpclett.1c03094
- 23.
- “Computational Studies on Reaction Mechanisms and Origin of Stereoselectivity in the [1,3]-Rearrangement
of Ene-Aldimines”
Momiyama, N.; Honda, Y.; Suzuki, T.; Jongwohan, C.
Asian JOC
2021,
10, 2205-2112 (Special Issue for Organocatalysis, invitation only).
DOI: 10.1002/ajoc.202100302
- 22.
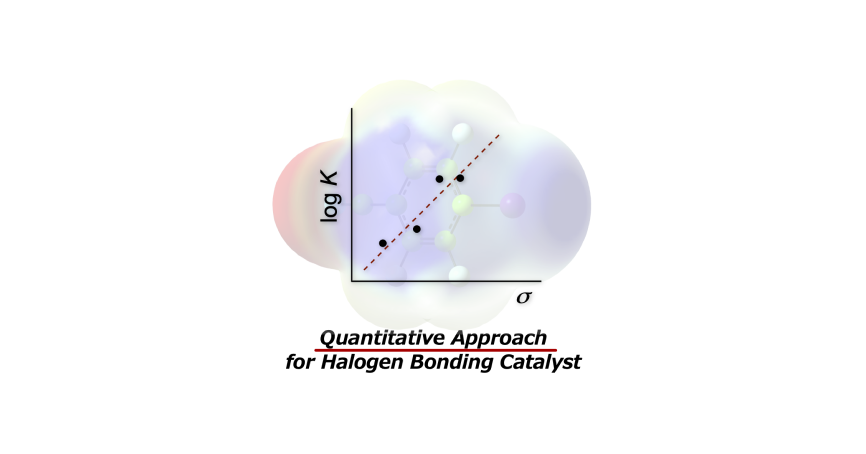
- “Correlations between Substituent Effects and Catalytic Activities: A Quantitative Approach for the
Development of Halogen-Bonding-Driven Anion-Binding Catalysts”
Momiyama, N.; Izumiseki, A.; Ohtsuka, N.; Suzuki, T.
ChemPlusChem
2021, 86, 913-919 (Special Issue for ISXB-4,
invitation only).
DOI: 10.1002/cplu.202100147
- 21.
- “Brønsted Acid-Initiated Formal [1,3]-Rearrangement Dictated by β-Substituted Ene-Aldimines”
Jongwohan, C.; Honda, Y.; Suzuki, T.; Fujinami, T.; Adachi, K.; Momiyama, N.
Org. Lett. 2019, 21, 4991-4995.
DOI:
10.1021/acs.orglett.9b01533
- 20.
- “Molecular Design of a Chiral Brønsted Acid with Two Different Acidic Sites: Regio-, Diastereo-, and
Enantioselective Hetero-Diels–Alder Reaction of Azopyridinecarboxylate with Amidodienes Catalyzed by Chiral
Carboxylic Acid–Monophosphoric Acid”
Momiyama, N.; Tabuse, H.; Noda, H.; Yamanaka, M.; Fujinami, T.; Yamanishi, K.; Izumiseki, A.; Funayama, K.;
Egawa, F.; Okada, S.; Adachi, H.; Terada, M.
J. Am. Chem. Soc. 2016, 138, 11353-11359.
DOI: 10.1021/jacs.6b07150
Highlighted in Synfacts 2016, 12, 1198.
DOI: 10.1055/s-0036-1589421
- 19.
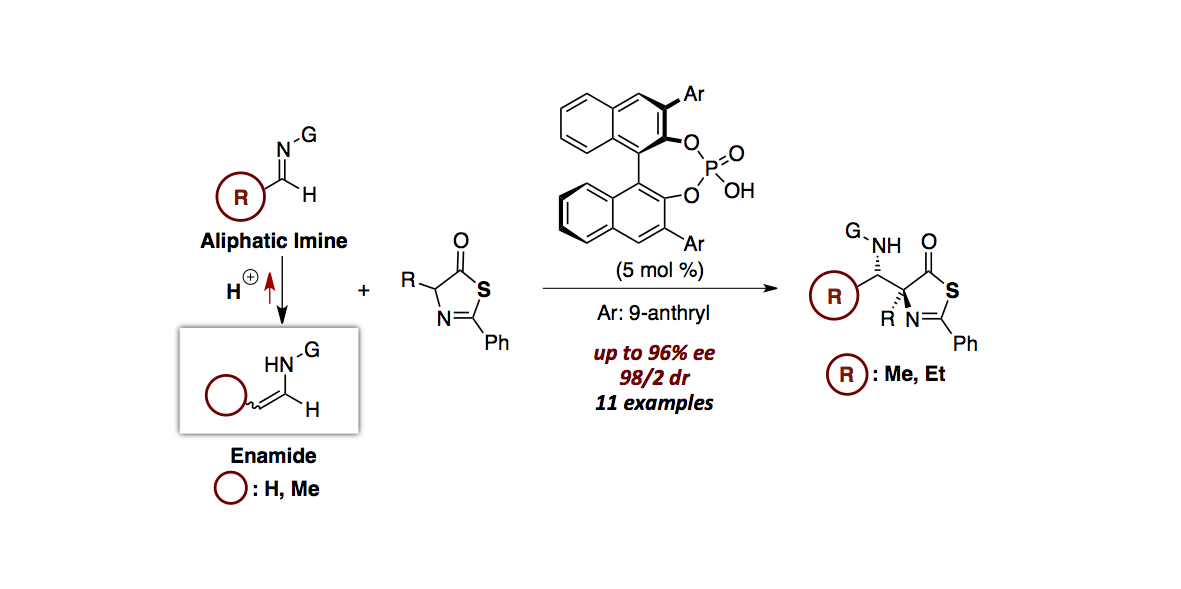
- “Chiral Phosphoric Acid-Catalyzed Diastereo- and Enantioselective Mannich-Type Reaction between Enamides
and Thiazolones”
Kikuchi, J.; Momiyama, N.; Terada, M.
Org. Lett. 2016, 18,
2521-2523.
DOI:
10.1021/acs.orglett.6b00857
- 18.
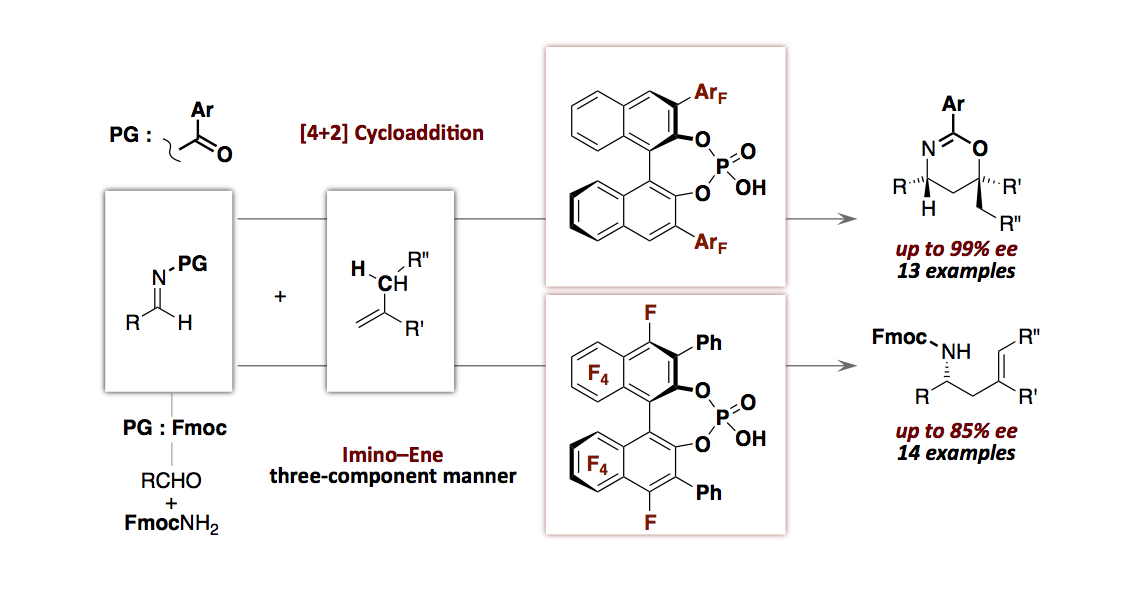
- “Perfluorinated Aryls in the Design of Chiral Brønsted Acid Catalysts: Catalysis of Enantioselective [4+2]
Cycloadditions and Ene–Reactions of Imines with Alkenes by Chiral Mono-Phosphoric Acids with
Perfluoroaryls”
Momiyama, N.; Okamoto, H.; Kikuchi, J.; Korenaga, T.; Terada, M.
ACS Catal. 2016, 6,
1198-1204.
DOI:10.1021/acscatal.5b02136
Highlighted in Synfacts 2016, 12, 413.
DOI:10.1055/s-0035-1561838
- 17.
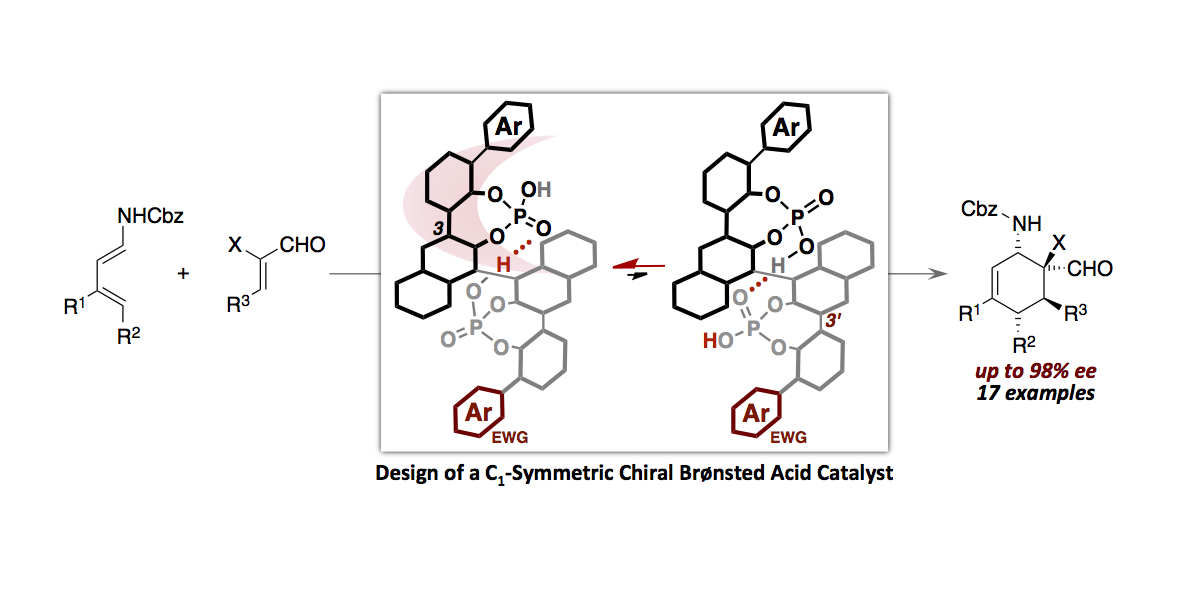
- “Hydrogen Bonds-Enabled Design of a C1-Symmetric Chiral Brønsted Acid Catalyst”
Momiyama, N. Funayama, K.; Noda, H.; Yamanaka, M.; Akasaka, N.; Ishida, S.; Iwamoto, T.; Terada, M.
ACS Catal. 2016, 6,
949-956.
DOI: 10.1021/acscatal.5b02079
Highlighted in Synfacts. 2016, 12, 308.
DOI: 10.1055/s-0035-1561676
- 16.
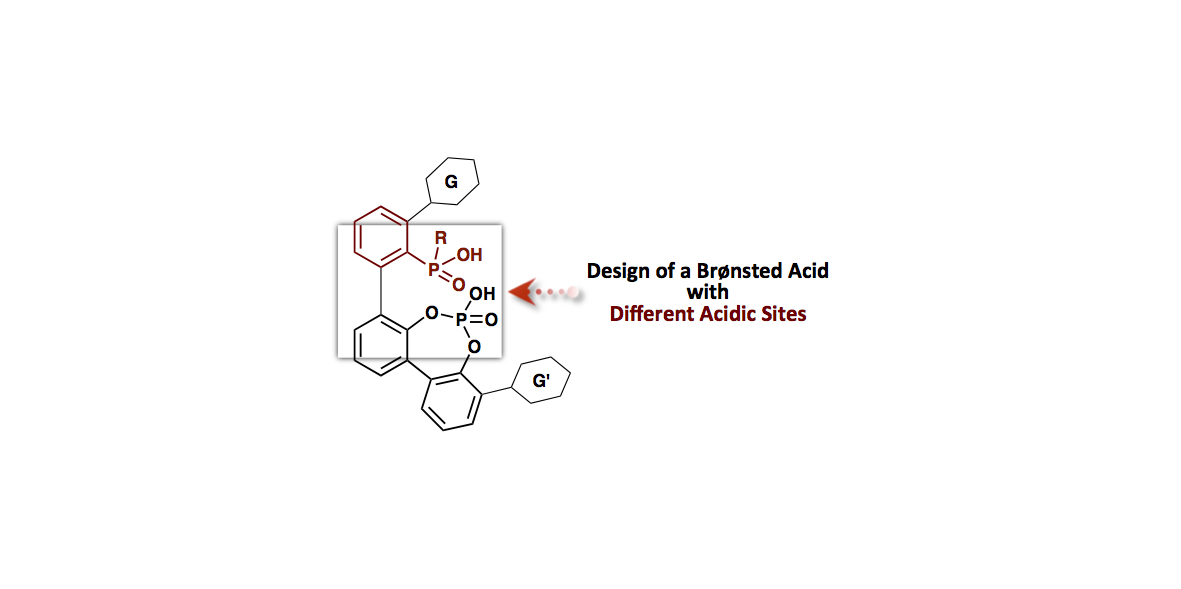
- “Design of a Brønsted Acid with Two Different Acidic Sites: Synthesis and Application of Aryl Phosphinic
Acid-Phosphoric Acid as a Brønsted Acid Catalyst”
Momiyama, N.; Narumi, T.; Terada, M.
Chem. Commun. 2015, 51, 16976-16979.
DOI: 10.1039/C5CC06787A
- 15.
- “Synthetic Method for 2,2’-Disubstituted Fluorinated Binaphthyl Derivatives and Application as Chiral
Source in Design of Chiral Mono-Phosphoric Acid Catalyst”
Momiyama, N.; Okamoto, H.; Shimizu, M.; Terada, M.
Chirality 2015, 27,
464-475 (Invited).
DOI: 10.1002/chir.22429
- 14.
- “Design of Chiral Bis-phosphoric Acid Catalyst Derived from (R)-3,3’-Di(2-hydroxy-3-arylphenyl)binaphthol: Catalytic Enantioselective
Diels-Alder Reaction of α,β-Unsaturated Aldehydes with Amidodienes”
Momiyama, N.; Konno, T.; Furiya, Y.; Iwamoto, T.; Terada, M.
J. Am. Chem. Soc. 2011, 133, 19294-19297.
DOI: 10.1021/ja2081444
- 13.
- “Chiral Brønsted Acid Catalysis for Enantioselective Hosomi-Sakurai Reaction of Imines with
Allyltrimethylsilanes”
Momiyama, N.; Nishimoto, H.; Terada, M.
Org. Lett. 2011, 13,
2126-2129.
DOI: 10.1021/ol200595b
- 12.
- “Chiral Phosphoric Acid-Governed Anti-Diastereoselective and Enantioselective Hetero-Diels-Alder Reaction
of Glyoxylate”
Momiyama, N.; Tabuse, H.; Terada, M.
J. Am. Chem. Soc. 2009, 131, 12882-12883.
DOI: 10.1021/ja904749x
- 11.
- “Enantioselective Activation of Aldehydes by Chiral Phosphoric Acid Catalysts in an Aza-Ene-type Reaction
between Glyoxylate and Enecarbamate”
Terada, M.; Soga, K.; Momiyama, N.
Angew. Chem., Int. Ed. 2008, 47, 4122-4125.
DOI: 10.1002/anie.200800232
- 10.
- “Synthesis of Acyclic α,β-Unsaturated Ketones via Pd(II)-Catalyzed
Intermolecular Reaction of Alkynamides and Alkenes”
Momiyama, N.; Kanan, M. W.; Liu, D. R.
J. Am. Chem. Soc. 2007, 129, 2230-2231.
DOI: 10.1021/ja068886f
- 9.
- “Diastereo- and Enantioselective Synthesis of Nitroso Diels-Alder-type Bicycloketones Using Dienamine:
Mechanistic Insight into Sequential Nitroso Aldol/Michael Reaction and Application for Optically Pure
1-Amino-3,4-diol Synthesis”
Momiyama, N.; Yamamoto, Y.; Yamamoto, H.
J. Am. Chem. Soc. 2007, 129, 1190-1195.
DOI: 10.1021/ja066037m
- 8.
- “Metal-Induced Reaction of O-Nitroso Aldol Product”
Morales, M. R.; Momiyama, N.; Yamamoto, H.
Synlett 2006, 705-706.
DOI: 10.1055/s-2006-933123
- 7.
- “Brønsted Acid Catalysis of Achiral Enamine for Regio- and Enantioselective Nitroso Aldol Synthesis”
Momiyama, N.; Yamamoto, H.
J. Am. Chem. Soc. 2005, 127, 1080-1081.
DOI: 10.1021/ja0444637
- 6.
- “O-Nitroso Aldol Synthesis. Catalytic Enantioselective Route to α-Aminooxy Carbonyl Compounds via Enamine
Intermediate”
Momiyama, N.; Torii, H.; Saito, S.; Yamamoto, H.
Proc. Natl. Acad. Sci. U.S.A. 2004, 101, 5374-5378.
DOI: 10.1073/pnas.0307785101
- 5.
- “Enantioselective Tandem O-Nitroso Aldol/Michael Reaction”
Yamamoto, Y; Momiyama, N.; Yamamoto, H.
J. Am. Chem. Soc. 2004, 126, 5962-5963.
DOI: 10.1021/ja049741g
- 4.
- “Enantioselective O- and N-Nitroso Aldol Synthesis of Tin Enolates. Isolation of Three BINAP-Silver
Complexes and Their Role of in Regio- and Enantioselectivity”
Momiyama, N.; Yamamoto, H.
J. Am. Chem. Soc. 2004, 126, 5360-5361.
DOI: 10.1021/ja039103i
- 3.
- “Catalytic Enantioselective Synthesis of α-Aminooxy and α-Hydroxy Ketone Using Nitrosobenzene”
Momiyama, N.; Yamamoto, H.
J. Am. Chem. Soc. 2003, 125, 6038-6039.; 2004, 126, 6498.
DOI: 10.1021/ja0298702, 10.1021/ja040805x
- 2.
- “Simple Synthesis of α-Hydroxyamino Carbonyl Compounds: New Scope of the Nitroso Aldol Reaction”
Momiyama, N.; Yamamoto, H.
Org. Lett. 2002, 4,
3579-3582.
DOI: 10.1021/ol026443k
- 1.
- “Lewis Acid Promoted, O-Selective, Nucleophilic Addition of Silyl Enol Ethers to N=O bonds”
Momiyama, N.; Yamamoto, H.
Angew. Chem., Int. Ed. 2002, 41, 2986-2988.; 3313.
DOI:
10.1002/1521-3773(20020816)41:16<2986::AID-ANIE2986>3.0.CO;2-F,
10.1002/1521-3773(20020916)41:18<3313::AID-ANIE11113313>3.0.CO;2-X

- 7.
- “三中心ハロゲン結合を基盤とする分子性触媒の創成−有機配位子と非金属活性中心の組み合わせ−”
椴山 儀恵
2022年58巻10号p.943-947.
https://doi.org/10.14894/faruawpsj.58.10_948
- 6.
- “Noncovalent Interactions in the Design of Chiral Brønsted Acid Catalysts”
Momiyama, N.
Chapter 10, pp. 209-231. In Noncovalent Interactions in Catalysis, Mahmudov,
K. T.; Kopylovich, M. N.; Guedes da Silva, M-F. C.; Pombeiro, A-J. L. Eds. The Royal Society of Chemistry
2019.
https://pubs.rsc.org/en/Content/eBook/978-1-78801-468-7
- 5.
- “Enantioselective Synthesis of Amines by Chiral Brønsted Acid Catalysts”
Terada, M.; Momiyama, N.
pp. 75-129, Chiral Amine Synthesis, Nugent, T. C. Ed. 2010.
- 4.
- “Development of Organocatalysis based on the Molecular Design of Pyrrolidine-Brønsted Acid”
Saito, S.; Momiyama, N.; Yamamoto, H.
Yuki Gosei Kagaku Kyokaishi, 2008, 66, 774-784.
- 3.
- “Brønsted Acid Catalyzed Nitroso Aldol Reaction”
Momiyama, N.; Yamamoto, H.
Chapter 8, pp. 85-105. CMC books, Shibasaki, M. Ed. 2006.
- 2.
- “Nitrosobenzene”
Momiyama, N.
Electronic Encyclopedia of Reagents for Organic Synthesis.
- 1.
- “Rich Chemistry of Nitroso Compounds”
Yamamoto, H.; Momiyama, N.
Chem. Commun. 2005, 3514-3525.

- 7.
- “ピリジン誘導体担持高分子及びこれを用いた転移反応”
 儀恵、山西克典、渡辺拓 特開2019−189808(P2019−189808A)
儀恵、山西克典、渡辺拓 特開2019−189808(P2019−189808A)
- 6.
- “環上に置換基を有する含窒素環状化合物の製造方法”
 儀恵、泉関督人 特開2018−104361(P2018-104361A)
儀恵、泉関督人 特開2018−104361(P2018-104361A)
- 5.
- “Process for preparation of bisphosphosphates as catalysts for asymmetric reactions”
Terada, M.; Momiyama, N.; Konno, T.
PCT Int. Appl. 2011, WO 2011111677 A1 20110915.
- 4.
- “Process of making alpha-aminooxyketone/alpha-aminooxyaldehyde and alpha-hydroxyketone
/alpha-hydroxyaldehyde compounds and a process making reaction products from cyclic alpha,beta-unsaturated
ketone substrates and nitroso substrates.”
Momiyama, N.; Torii, H.; Saito, S.; Yamamoto, H.; Yamamoto, Y.
U.S. Pat. Appl. Publ. 2007, US20070037973 A1
20070215.
- 3.
- “Process of making α-aminooxyketone/α-aminooxyaldehyde compounds and α-hydroxyketone/α-hydroxyaldehyde
compounds and a process of making reaction products from cyclic α,s,s-unsaturated ketone substrates and
nitroso substrates.”
Momiyama, N.; Torii, H.; Saito, S.; Yamamoto, H.; Yamamoto, Y.
PCT Int. Appl. 2005, WO 2005090294 A2 20050929.
- 2.
- “Preparation of aminooxy compounds, hydroxy amines, and hydroxy ketones, and catalysis for it.”
Yamamoto, H.; Momiyama, N.
Jpn. Kokai Tokkyo Koho. 2004, JP 2004115446 A
20040415.
- 1.
- “Preparation of hydroxyamines and/or aminooxy compounds, and Lewis acid-containing catalysts for the
regioselecive nucleophilic addition reaction.”
Yamamoto, H.; Momiyama, N.; Yanagisawa, A.
Jpn. Kokai Tokkyo Koho. 2003, JP 2003313158 A
20031106.



 儀恵、山西克典、渡辺拓 特開2019−189808(P2019−189808A)
儀恵、山西克典、渡辺拓 特開2019−189808(P2019−189808A) 儀恵、泉関督人 特開2018−104361(P2018-104361A)
儀恵、泉関督人 特開2018−104361(P2018-104361A)