Research
Developing Functions and Principles That Bring Innovation and Breakthrough in Material Science
There exists fundamental principles that rationalize the diverse functions in materials. Finding new principles has brought significant advances and innovations in both fundamental and applied science. We focus on open-shell molecules such as radicals and magnetic metal complexes and investigate their unique functions. We dream to find innovative concepts and principles that enable to create new field in science.
What we do is very simple: preparing new molecules, examining their structures, properties, and electronic states…. We believe it is important to try sincerely to rationalize unexpected phenomena or unusual data we face for developing novel science.
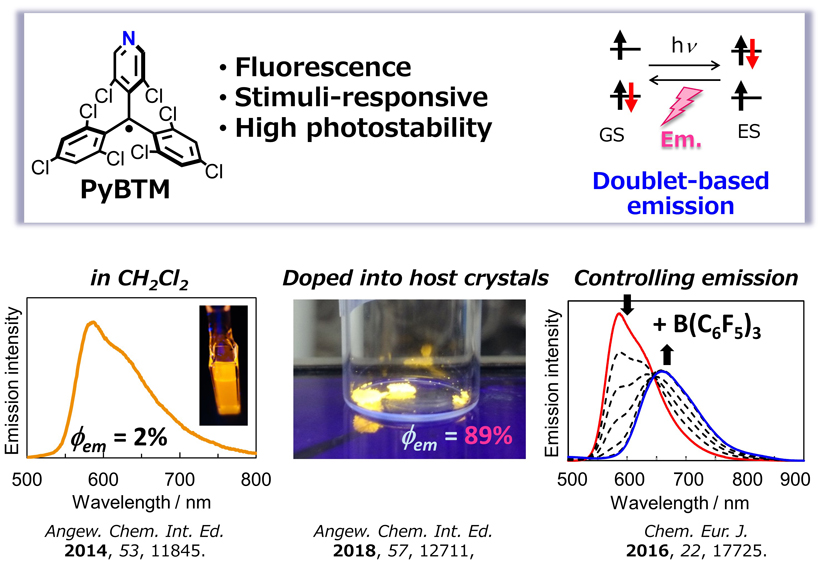
Luminescent Radicals
Radicals are known as non-emissive species and behave as quenchers of luminophore. The radicals tend to decompose upon photoirradiation. Indeed, the luminescent properties of the radicals are not well established and remain undisclosed. We have developed a luminescent stable radical PyBTM, and revealed that PyBTM is highly stable upon photoirradiation. The emission quantum yield (Φem) of PyBTM in the solution state is typically 1~3%, while that in the solid matrix increases up to 89%. PyBTM is a promising candidate for developing luminescent properties based on the doublet (multiplet) state.
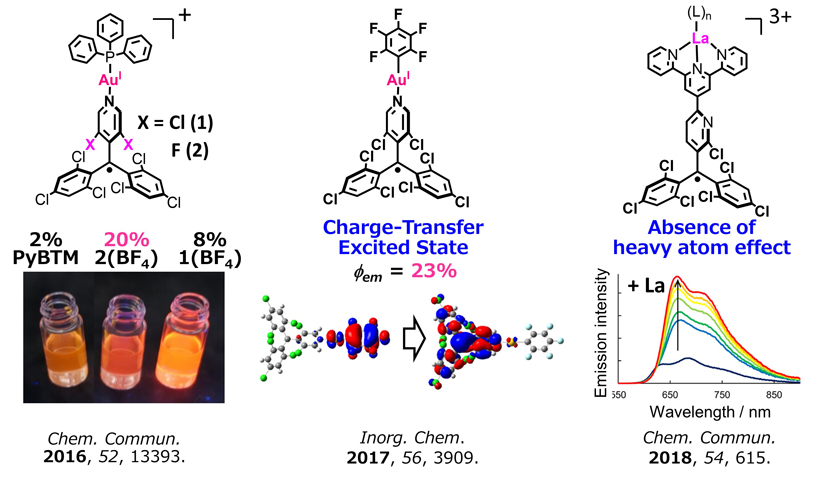
Enhanced Photofunctions of Radicals via Metal Complex Formation
To date, luminescent radicals have been shown to lose their emission characters upon coordination to metal ions. In contrast, we have revealed that the coordination to metal ions is an efficient strategy to enhance, vary, or integrate the photofunctions of the luminescent radicals. For example, Φem increases over 20% upon coordination of PyBTM to gold to form [AuI(PyBTM)(PPh3)](BF4) or [AuI(C6F5)(PyBTM). We have proposed that doublet-based fluorescence is free from heavy atoms that typically quench the fluorescence of conventional fluorophores. We believe coordination chemistry is a powerful method to develop novel photofunctions of radicals.
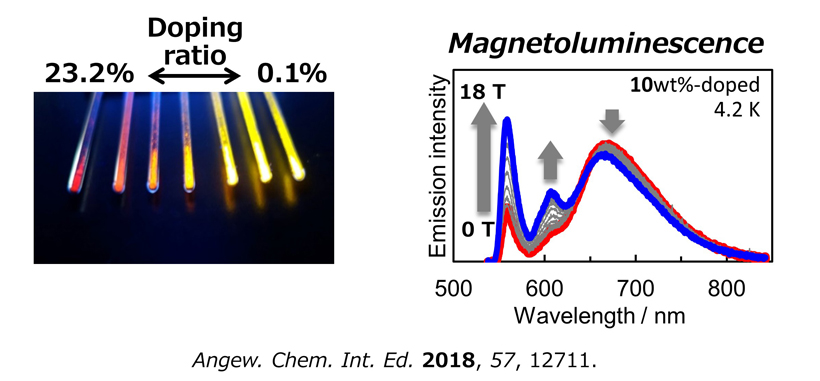
Spin-Luminescence Correlated Function on Radicals
Interplay between spin and luminescence enables to produce photofunctions that are unique to open-shell electronic system. We have shown that PyBTM doped into host molecular crystals displays distinct luminescence depending on the doping ratio. In particular, 10wt% doped sample exhibits magnetic field-controlled luminescence (i.e., magnetoluminescence) at liquid helium temperature; this is the first observation of magnetoluminescence in radicals. This phenomenon is attributed to interplay between spin and luminescence. We expect that magnetic field-induced intersystem crossing on a radical-pair that is transiently generated in the photoexcited state would play an important role in this unique magnetic field effect. These results indicate that luminescent radicals have a great potential to create novel properties that is difficult to achieve for conventional closed-shell luminescent molecules.
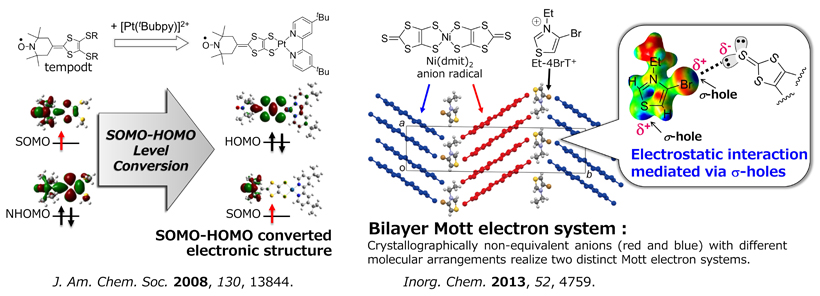
Controlling Frontier Orbitals and Molecular Arrangements of Radicals
Control of frontier orbitals: Radicals possessing SOMO-HOMO converted peculiar electronic structure enable to form another unpaired electron to be diradicals upon oxidation, and display unique chemical and physical properties that are not realized in conventional radicals and inorganic compounds. However, the candidates are much limited so far. We have revealed that tempo radical-bound dithiolene ligand (tempodt) coordinates to the metal ions to yield open-shell metal complexes with the SOMO-HOMO converted electronic structure. On the basis of this finding, we modulated the frontier orbitals of radicals with a variety of methods to achieve unique molecular functions, such as coexistence of 3d-spin and π-spins on a diradical (Inorg. Chem. 2013), controlling charge transfer direction (Inorg. Chem. 2012), spin-reconstructed proton-coupled electron transfer (J. Am. Chem. Soc. 2015), and a NIR-emissive radical showing acid-induced intramolecular electron transfer (J. Phys. Chem. C 2019).
Control of arrangement of molecules: σ-hole bond is an electrostatically-driven non-covalent intermolecular interaction. We aimed to realize novel electronic states via novel molecular arrangements by utilizing σ-hole bonds in radical-based molecular crystals. We have shown that molecular crystals composed of Ni(dmit)2 anion radicals form “bilayer Mott electron system”. Mott electron system is one of the strongly-correlated electron systems, and can be a origin of peculiar solid state properties such as superconductivity and quantum spin liquids. In bilayer Mott electron system, two kinds of Mott electron systems coexist in a crystal lattice. Such a peculiar electronic state has provided unusual physical properties such as coexistence of ferromagnetic short-range ordering and antiferromagnetic short-range ordering, gigantic magnetoresistance, etc. Novel and unique molecular arrangement promises unexpected physical properties.